History
Leishmaniasis has been the cause of great suffering and
death for hundreds of years.
Etiology
Leishmaniasis is the result of infection with intracellular protozoan parasites belonging to the genus Leishmania. The organisms are found in two morphologic forms during their life cycle. In humans and other mammalian hosts, they exist within macrophages as round to oval nonflagellated amastigotes and in the arthropod vectors (sandflies) the parasites exist as elongated flagellated promastigotes
About 30 species of sandflies can become infected when taking a blood meal from a reservoir host. In the old world genus Phlebotomus and in the new world genus Lutzomia are responsible for transmitting the disease. Sandflies are small mosquito-like insects 1.5 to 4 mm in length and their small size allows them to pass through ordinary mesh screens and mosquito netting.
Hosts are infected humans, wild animals, such as rodents, and domestic animals, such as dogs. Most leishmaniases are zoonotic (transmitted to humans from animals), and humans become infected only when accidentally exposed to the natural transmission cycle. However, in the anthroponotic forms (those transmitted from human to human through the sandfly vector), humans are the sole reservoir host.
Classification
Leishmaniasis presents itself in humans in four different forms with a broad range of clinical manifestations. All forms can have devastating consequences. Similar to Hansen disease, leishmaniasis is a disease in which the clinical diversity reflects a complex interplay between the virulence of the infecting species and the host's immune response. At one extreme, localized cutaneous disease demonstrates a vigorous immune response, with most cases resolving without intervention. This form of disease exhibits a helper T-cell subtype 1 (TH1) immune response, with interleukin 2, interferon g, and interleukin 12 as the prominent cytokines that induce disease resolution. At the other extreme, with visceral or diffuse cutaneous disease, patients exhibit relative anergy to the Leishmania organism and have a prominent helper T-cell subtype 2(TH2) cytokine profile.
Visceral leishmaniasis (VL), also known as kala azar, is the most severe form of the disease, which, if untreated, has a mortality rate of almost 100%. It is characterized by irregular bouts of fever, substantial weight loss, swelling of the spleen and liver, and anaemia.
Mucocutaneous leishmaniasis (MCL), or espundia, produces lesions which can lead to extensive and disfiguring destruction of mucous membranes of the nose, mouth and throat cavities.
Cutaneous leishmaniasis (CL) can produce large numbers of skin ulcers—as many as 200 in some cases—on the exposed parts of the body, such as the face, arms and legs, causing serious disability and leaving the patient permanently scarred. Diffuse cutaneous leishmaniasis (DCL) never heals spontaneously and tends to relapse after treatment. The cutaneous forms of leishmaniasis are the most common and represent 50-75% of all new cases.
Epidemiology
Geographic Distribution
Increased Prevalence
Leishmania/HIV
Co-infection
Leishmania/HIV
co-infection is emerging as an extremely serious, new disease and it is
increasingly frequent. Leishmania/HIV co-infections are considered a real
threat, especially in south-western Europe
where between 25% and 70% of adult VL cases are related to HIV
and where 1.5%to 9% of AIDS cases suffer form newly acquired or
reactivated VL.
Intravenous drug users have been identified as the main population at
risk.
It is anticipated that the number of Leishmania/HIV co-infections will continue to rise in the coming years and there are indications that cases are no longer restricted to endemic areas. The overlapping geographical distribution of VL and AIDS is increasing due to two main factors: the spread of the AIDS pandemic in suburban and rural areas of the world, and the simultaneous spread of VL from rural to suburban areas.There are important clinical, diagnostic, chemotherapeutic, epidemiological and economic implications of this trend.
This
duo of diseases produces cumulative deficiency of the immune response since Leishmania
parasites and HIV destroy the same cells, exponentially increasing disease
severity and consequences. VL is considered a major contributor to a fatal
outcome in co-infected patients. Lately, however, use of tritherapy, where it is
available, has improved the prognosis for Leishmania/HIV cases.
Specific Problems
The
main alternative drugs include pentamidine, amphotericin B and amphotericin B
encapsulated in liposomes. This encapsulation reduces the occurrence of
side-effects, but relapses still occur and the drug remains extremely expensive.
Epidemiological Changes
Clinical
Patterns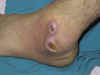
Old world cutaneous leishmaniasis
begins as a small erythematous papule, which may appear immediately after the
bite of the sandfly but usually appears 2 to 4 weeks later. One or more lesions
occur on unclothed part of the body. Lesions may be associated with
sporotrichotic spread and usually heal spontaneously. The sequence of nodule,
crusting, ulceration and healing with scar formation is common to all the
self-healing sores.
Clinical variants of CL:
Diffuse cutaneous leishmaniasis
(DCL): Analogous to
lepromatous leprosy, individuals with DCL fail to mount a cell-mediated immune
response to the Leishmania parasite. Consequently, patients develop multiple
widespread cutaneous papules and nodules and are anergic to leishmanin skin
testing.
Recidivans cutaneous leishmaniasis
(RCL): A relatively uncommon
clinical
variant of leishmaniasis, RCL presents as a recurrence of lesions at the
site of apparently healed disease years after the original infection.Typically,
RCL lesions occur on the face, and RCL presents as an enlarging papule, plaque,
or coalescence of papules that heals with central scarring. Relentless expansion
at the periphery may cause significant facial destruction similar to the lupus
vulgaris variant of cutaneous tuberculosis.
Post kala azar dermal leishmaniasis (PKDL): Endemic to India and the Sudan, this form of leishmaniasis develops months to years after the patient’s recovery from visceral leishmaniasis. Cutaneous lesions demonstrate great variability, ranging from hypopigmented macules to erythematous papules and from nodules to plaques. As in leprosy, the wide clinical spectrum of PKDL reflects the immune response of the individual to the Leishmania organism. Lesions may be numerous and persist for decades. Isolated parasites from the lesions are identical to those causing the original visceral disease.
Diagnosis
Differential diagnosis of LCL is
extensive and includes impetigo, pyoderma gangrenosum, deep fungal infection,
mycobacterial infection, sarcoidosis, and squamous cell carcinoma. In the
endemic areas the clinical diagnosis is not difficult but the definite diagnosis
rests on finding parasites in the skin. Usually, making a smear of material from
the sore and staining it with Wright’s, Giemsa or Leishman’s stain on a
microscope slide best achieve this. In addition, Polymerase chain reaction (PCR)
is one of the newest techniques used to identify leishmaniasis and shows
significant promise as a method applicable for both detection and speciation.
Most research laboratories have reported higher sensitivity and specificity with
PCR than with other currently available diagnostic methods.
Treatment
The treatment of leishmaniasis
depends on the clinical form of the disease. For 50 years, the mainstay of
antileishmanial therapy has been pentavalent
antimony (sodium stibogluconate or meglumine antimonate).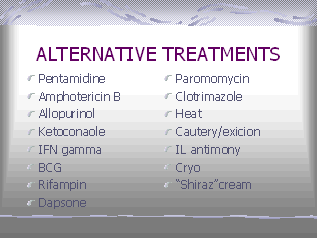
Other measures include freezing,
local heat, oral ketoconazole, rifampicin and topical paromomycin. Surgical
excision usually is not recommended because of the risk of relapse and cosmetic
disfigurement.
To date, no vaccines are available
commercially.
The World Health Organization Response
WHO has set the following objectives:
1. To provide early diagnosis
and prompt treatment;
2. To control the sandfly
population through residual insecticide spraying of houses
and through the use of insecticide-impregnated bed nets;
3. To provide health education
and produce training materials;
4. To detect and contain
epidemics in the early stages;
5. To provide early diagnosis
and effective management for Leishmania/HIV coinfections.
Because of the anticipated substantial
increase in Leishmania/HIV co-infections, they are among the priorities
for WHO's Department of Communicable Disease Surveillance and Response (CSR).
The evolution of Leishmania/HIV
co-infection is being closely monitored by extending the geographic coverage of
the surveillance network and by improving case reporting. WHO encourages active
medical surveillance, especially in south-western Europe, of intravenous drug
users, the main population at risk. Finally, because case notification of
leishmaniasis is compulsory in only 40 of the 88 endemic countries, WHO strongly
suggests the remaining 48 endemic countries follow suit.
Summery
Leishmaniasis
is a disease caused by protozoa, and it affects as many as 12 million people
worldwide, with 1.5-2 million new cases each year. Transmitted by the bite of a
sandfly, the clinical spectrum of leishmaniasis ranges from a self-resolving
cutaneous ulcer, to a mutilating mucocutaneous disease, to a fatal systemic
illness. The global incidence of this infectious disease has been increasing
during recent years because of increased international travel, human alteration
of vector habitats, and concomitant factors that result in increased
susceptibility such as HIV infection and malnutrition. Many Leishmania species
transmit the disease, and the clinical spectrum, although once believed to be
predictable, continues to evolve. Diagnosis may be difficult because of the
small size of the protozoa sequestered within macrophages of the skin, bone
marrow, and reticuloendothelial system. Therapy has long been a challenge for
the more severe forms of the disease and is made more difficult by the emergence
of drug resistance. No effective vaccine
is available for leishmaniasis.
Bibliography
1. I Cruz, M A Morales, I Noguer, A Rodríguez, J Alvar Leishmania in discarded syringes from intravenous drug users. Lancet 2002; 359: 1124-25
2. ADM Bryceson, RJ Hay. Parasitic worms and protozoa. In : Rook/Wilkinson/Ebling Textbook of dermatology.6th ed.1998
3. SN Klaus, S Frankenburg. In: Fitzpatrick’s Dermatology in general medicine. 5th ed.1999